EYLEA HD Delivers Three-Year Vision Gains, Offering Longer Dosing for Patients
Regeneron’s EYLEA HD Study Achieves Promising Results
Regeneron Pharmaceuticals has revealed compelling three-year results from an extended study of EYLEA HD (aflibercept) Injection 8 mg, targeting wet age-related macular degeneration (wAMD). Presented at a recent annual meeting, the findings indicate that a significant number of patients were able to sustain their visual and anatomical improvements. Notably, 24% of patients required injections as infrequently as twice a year. Additionally, those who switched from a fixed two-month schedule to EYLEA HD also demonstrated ongoing benefits, with 79% and 43% able to extend their dosing intervals to at least three and four months, respectively. The safety profile was in line with prior studies, with common side effects including cataract and retinal hemorrhage. These results further validate EYLEA HD’s effectiveness and underscore its potential to ease the treatment burden for older patients.
Positive Highlights from the Study
- Over three years, most patients treated with EYLEA HD maintained visual and anatomical improvements, supporting the drug’s effectiveness.
- The study suggests that 77% of patients achieved dosing intervals of three months or more, significantly lowering the number of treatments needed.
- The Phase 3 PULSAR trial’s outcomes add to the existing evidence backing EYLEA HD’s efficacy, solidifying its status in the wet AMD treatment landscape.
- The safety profile remained stable and consistent with the original EYLEA, offering reassurance to healthcare providers and patients alike.
Potential Concerns
- Potential side effects, including cataract, retinal hemorrhage, and increased intraocular pressure, could raise safety concerns among patients and healthcare professionals.
- Regeneron faces ongoing regulatory responsibilities that may complicate its research and clinical programs, signaling possible challenges in developing EYLEA HD commercially.
- Mention of pending civil proceedings with the U.S. Department of Justice introduces a degree of uncertainty regarding the company’s future operations and financial health.
Frequently Asked Questions
What are the main findings of the EYLEA HD three-year study?
The study found that most patients were able to sustain visual and anatomical improvements while enjoying extended dosing intervals.
How did EYLEA HD influence dosing schedules for patients?
Many patients were able to extend their dosing intervals to three, four, five, or even six months, thereby reducing the frequency of treatments.
What were the benefits of switching from EYLEA to EYLEA HD?
Patients who switched to EYLEA HD maintained their visual improvements while benefitting from fewer injections.
What does the safety profile of EYLEA HD look like after three years?
The safety profile has shown consistency with established effects, including ocular treatment-related adverse events, paralleling findings from EYLEA.
Which conditions does EYLEA HD treat?
EYLEA HD is approved for treating Wet Age-Related Macular Degeneration (wAMD), Diabetic Macular Edema (DME), and Diabetic Retinopathy (DR).
Disclaimer: This is an AI-generated summary of a press release distributed by GlobeNewswire. The model used may contain inaccuracies. For the complete release, click here.
$REGN Congressional Stock Trading
In the past six months, Congress members have engaged in $REGN stock transactions on six occasions, with no purchases and all being sales.
Here’s a detailed look at recent congressional trading activity related to $REGN stock:
To monitor congressional stock trading, visit Quiver Quantitative’s congressional trading dashboard.
$REGN Insider Trading Activity
Company insiders have traded $REGN stock 58 times over the past six months, with no purchases and all being sales.
Highlights from insider trading activity for $REGN include:
- CHRISTINE A POON: 0 purchases, 7 sales of 10,838 shares, totaling approximately $12,552,284.
- ANDREW J MURPHY (EVP Research): 0 purchases, 18 sales of 6,363 shares, totaling about $7,337,504.
- CHRISTOPHER R. FENIMORE (SVP Finance & CFO): 0 purchases, 10 sales of 5,680 shares, amounting to roughly $6,846,262.
- MARION MCCOURT (EVP Commercial): 0 purchases, 2 sales of 2,000 shares, for about $2,023,340.
- BONNIE L BASSLER sold 756 shares for approximately $884,520.
- ARTHUR F RYAN: 0 purchases, 20 sales of 100 shares, totaling around $117,868.
For more information on insider transactions, visit Quiver Quantitative’s insider trading dashboard.
$REGN Hedge Fund Activity
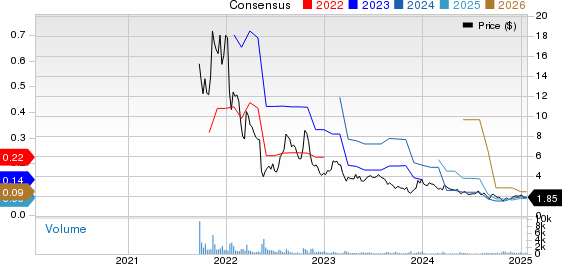
In the most recent quarter, 598 institutional investors have increased their positions in $REGN stock, while 666 have reduced theirs.
Significant recent moves include:
- JPMORGAN CHASE & CO sold 787,815 shares (-9.5%) in Q3 2024, representing approximately $828,182,640.
- WELLINGTON MANAGEMENT GROUP LLP sold 621,182 shares (-58.5%) in Q3 2024, valued at about $653,011,365.
- AMUNDI added 487,489 shares (+45.8%) in Q4 2024, worth an estimated $347,253,039.
- PRICE T ROWE ASSOCIATES INC /MD/ added 342,429 shares (+36.7%) in Q3 2024, for an estimated $359,975,061.
- CAPITAL WORLD INVESTORS sold 340,280 shares (-7.3%) in Q3 2024, valued at approximately $357,715,947.
- VANGUARD GROUP INC added 326,320 shares (+3.6%) in Q3 2024, estimated at $343,040,636.
- FRANKLIN RESOURCES INC added 315,689 shares (+16.4%) in Q3 2024, worth about $331,864,904.
To track hedge funds’ stock portfolios, visit Quiver Quantitative’s institutional holdings dashboard.
Full Release
After three years of treatment with EYLEA HD, most patients have successfully maintained their visual and anatomic improvements
while benefiting from extended dosing options, with some patients requiring injections only twice a year
: 77%, 58%, 40%, and 24% achieved their last assigned dosing intervals of ≥3, ≥4, ≥5, and 6 months, respectively
Following a switch to EYLEA HD from a fixed two-month dosing schedule with EYLEA
®
(aflibercept) Injection 2 mg, most patients showed pronounced and sustained improvements in vision and anatomy while rapidly increasing their dosing intervals: 79%, 43%, and 16% achieved last assigned dosing intervals of ≥3, ≥4, and ≥5 months, respectively
The results further enhance earlier findings demonstrating EYLEA HD’s capability to extend dosing intervals, including previously reported data in diabetic macular edema where 88%, 68%, 48%, and 28% of patients achieved similar improvements over three years.
Regeneron Unveils Promising Long-Term Data for EYLEA HD Treatment in Eye Disease
TARRYTOWN, N.Y., Feb. 08, 2025 (GLOBE NEWSWIRE) — Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) has announced encouraging three-year results from an extension study on EYLEA HD (aflibercept) Injection 8 mg in patients with wet age-related macular degeneration (wAMD). These findings were shared at the virtual Angiogenesis 2025 annual meeting, adding to the growing body of evidence supporting this treatment.
The study showed that patients who had previously participated in the Phase 3 PULSAR trial mostly maintained the vision and anatomical improvements they achieved over the course of two years. Notably, many experienced considerably longer intervals between treatments. Patients who transitioned from EYLEA (aflibercept) Injection 2 mg to EYLEA HD at the start of year three also preserved their vision gains while enjoying fewer required injections over the study’s duration.
“Older patients with wet age-related macular degeneration often need help getting to appointments. Easing their treatment burden can significantly enhance their quality of care,” explained W. Lloyd Clark, M.D., from the Palmetto Retinal Center and Assistant Clinical Professor of Ophthalmology at the University of South Carolina School of Medicine. He emphasized the significance of the new data, noting that a substantial number of patients managed to maintain their visual and anatomical improvements with just two doses each year.
During the PULSAR study, EYLEA HD patients initially followed a dosing schedule of 3 or 4 months after receiving three initial monthly doses. If they met set criteria, their dosing intervals could be modified throughout the trial. Remarkably, it was previously reported that 88% of EYLEA HD patients maintained 3-month intervals at the end of two years. Those who completed the optional extension study found:
- Nearly 60% recorded a last assigned dosing interval of at least 4 months, while 40% and 24% achieved intervals of at least 5 and 6 months, respectively, at the end of the three-year period.
- Vision and anatomical enhancements seen by year two persisted through year three, showing continued improvement in retinal thickness.
Participants in the PULSAR comparator group received EYLEA as a fixed 2-month dosing regimen for 96 weeks. Those who completed the extension study (n=186) switched to EYLEA HD and maintained their improvements, with 79% and 43% achieving dosing intervals of at least 3 and 4 months, respectively, at week 156.
The safety profile of EYLEA HD remained consistent with earlier studies over the three years. Noteworthy ocular treatment-related side effects occurring in over 4% of patients included cataracts, retinal hemorrhage, vision reduction, vitreous floaters, and increased intraocular pressure. The rate of inflammation was reported at 2.4% for patients transitioning from EYLEA to EYLEA HD and 1.9% for those originally randomized to EYLEA HD.
The three-year data from the PHOTON trial on EYLEA HD for DME were disclosed last October at the American Academy of Ophthalmology annual meeting.
EYLEA HD, available as Eylea™ 8 mg in Europe and Japan, is jointly developed by Regeneron and Bayer AG. In the United States, Regeneron retains exclusive rights to EYLEA and EYLEA HD, while Bayer holds exclusive marketing rights outside the U.S., with both companies sharing profits from sales.
Overview of EYLEA HD Clinical Trials
PULSAR for wAMD and PHOTON for DME and diabetic retinopathy (DR) are pivotal, double-masked, active-controlled trials conducted across multiple international centers. In both trials, participants were divided into three treatment groups, receiving EYLEA HD at either 3 or 4-month intervals or standard EYLEA treatment every 2 months. Bayer sponsored the PULSAR trial, while Regeneron sponsored the PHOTON trial.
Patients assigned to EYLEA HD initially received three monthly doses. For EYLEA, the number of initial doses varied by trial. In the first year, EYLEA HD dosing intervals could temporarily reduce to every 2 months based on defined criteria, while fixed dosing was maintained in other groups throughout the two-year duration.
All trial participants had the opportunity to join a follow-up extension beginning at week 96, allowing them to receive EYLEA HD through week 156. Those originally on EYLEA in PULSAR transitioned to EYLEA HD with a newly assigned 3-month interval. Adjustments to dosing schedules were made based on clinical guidelines, with a minimum of 2 months and a maximum of 6 months between doses.
Understanding wAMD and Diabetic Eye Disease
wAMD is a condition typically found in older individuals where abnormal blood vessels grow beneath the retina, leading to fluid leakage which can harm vision. Approximately 1.4 million Americans suffer from this disease.
Diabetic retinopathy (DR) is marked by damage to retinal blood vessels due to diabetes complications, often starting without symptoms. This condition can progress to proliferative diabetic retinopathy (PDR), where abnormal vessels lead to serious vision issues.
Diabetic macular edema (DME) can happen at any stage of DR, causing fluid leakage and vision problems. In the U.S., around 1.5 million adults are diagnosed with DME, while 6 million have DR without DME.
IMPORTANT SAFETY INFORMATION AND INDICATIONS
INDICATIONS
EYLEA HD (aflibercept) Injection 8 mg is a prescription medication approved for treating patients with wet age-related macular degeneration (AMD), diabetic macular edema (DME), and diabetic retinopathy (DR).
EYLEA (aflibercept) Injection 2 mg is also approved for treating patients with wet age-related macular degeneration (AMD) as well as other forms of macular edema.
Understanding EYLEA and EYLEA HD: Key Safety Information
Retinal diseases, such as Retinal Vein Occlusion (RVO) and Diabetic Macular Edema (DME), are treated with EYLEA and EYLEA HD, which contain aflibercept. These treatments aim to improve vision in patients suffering from various eye conditions.
Essential Safety Information
- EYLEA HD and EYLEA are injected directly into the eye. Patients should avoid these treatments if they have an eye infection, pain, redness, or allergies to ingredients in EYLEA HD or EYLEA, including aflibercept.
- Injections can lead to infections, retinal detachment, and, in rare cases, serious inflammation of blood vessels in the retina. Contact your doctor immediately if you or your child (being treated for Retinopathy of Prematurity) experience eye pain, light sensitivity, or vision changes post-injection.
- Temporary eye pressure increases may occur within an hour of the injection, with sustained increases possible after multiple treatments. Monitoring by your doctor is recommended post-injection.
- Extensive monitoring for Retinopathy of Prematurity (ROP) is essential for infants receiving EYLEA treatment.
- A unique but rare risk is the potential for serious side effects, including blood clots that may lead to heart attacks or strokes.
- Common side effects for EYLEA HD include cataracts, eye redness, increased eye pressure, discomfort, blurred vision, floaters, and bleeding at the back of the eye.
- Patients treated with EYLEA have reported eye redness, pain, cataracts, and similar symptoms to those noted for EYLEA HD.
- Pre-term infants with ROP may experience side effects like retinal separation and increased eye pressure. Adult side effects may also be relevant, though not all were confirmed in clinical studies.
- After receiving an EYLEA HD or EYLEA injection, you may notice temporary vision changes; avoid driving or operating machinery until your vision stabilizes.
- For further safety details, consult your doctor and review the complete Prescribing Information for EYLEA HD and EYLEA.
Reporting Side Effects
You can report negative side effects from prescription medications to the FDA. For more details, visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Complete Prescribing Information for EYLEA HD and EYLEA
About Regeneron
Regeneron (NASDAQ: REGN) is a prominent biotechnology firm committed to developing life-changing medicines. Founded by physician-scientists, Regeneron has consistently translated scientific innovation into effective treatments across various medical disciplines including eye diseases, cancer, and rare conditions.
The company advances scientific discovery and accelerates drug development through proprietary technologies like VelociSuite®, which creates human antibodies and bispecific antibodies. By utilizing insights from the Regeneron Genetics Center®, they explore new treatment opportunities to address an array of diseases.
To learn more about Regeneron, visit www.Regeneron.com or follow them on social media platforms including LinkedIn, Instagram, Facebook, and X.
Forward-Looking Statements
This document includes forward-looking statements that involve various risks and uncertainties regarding Regeneron’s future performance and operations. Phrases such as “anticipate,” “expect,” and “plan” aim to identify these projections, which may include uncertainties surrounding the success and therapeutic applications of products developed by Regeneron. Factors impacting these projections include regulatory approvals, market acceptance of products, and the management of supply chains for multiple treatments under development.
Regeneron’s Challenges and Opportunities: Navigating the Healthcare Landscape
The company faces a range of risks from market competition to regulatory changes.
Regeneron Pharmaceuticals is grappling with numerous external factors that could affect its business and future growth. These challenges include the strategies of pharmacy benefit management companies and government programs like Medicare and Medicaid. Changes in policies, regulations, and coverage decisions by these payers could influence Regeron’s ability to secure reimbursement for its products.
Additionally, the company must contend with competing drugs and product candidates that might offer better effectiveness or lower costs. This includes potential biosimilar versions of Regeneron’s products, which could further disrupt their market share.
As Regeneron advances its research and development initiatives, the company faces uncertainty regarding whether these efforts will yield replicable results across different studies. Furthermore, the path from initial research to clinical trials and finally to regulatory approval can be fraught with unexpected costs and challenges.
Financial projections are another point of concern. Regeneron must accurately forecast its expenses and revenues while adapting to any shifts in these underlying assumptions. Partnerships with companies like Sanofi and Bayer are currently pivotal; however, any changes or terminations in these agreements could pose risks to Regeneron’s strategic plans.
Regeneron’s operations are also susceptible to public health issues, such as outbreaks and pandemics, which can greatly impact the healthcare industry. Moreover, ongoing litigation, including civil proceedings initiated by the U.S. Department of Justice, adds another layer of complexity to the company’s outlook.
These risks can affect not just Regeneron’s operations but also its financial stability and overall market prospects. For a more comprehensive understanding of these risks, please refer to the company’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the year ending December 31, 2024.
Regeneron’s management emphasizes that any forward-looking statements are based on current beliefs and should not be deemed as guarantees of future performance. The company does not commit to updating these statements as new information arises.
For investors and interested parties, Regeneron has been proactive in utilizing its media and investor relations website alongside social media platforms to disseminate important updates about the company. Key information can be accessed on their media site (https://investor.regeneron.com) and their official LinkedIn page (https://www.linkedin.com/company/regeneron-pharmaceuticals).
This article was originally published on Quiver News; read the full story.
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.