Merck (MRK) announced that its phase III HYPERION study for the pulmonary arterial hypertension drug, Winrevair (sotatercept), achieved its primary endpoint. The study, which included newly diagnosed adult patients with intermediate or high-risk PAH, demonstrated a statistically significant reduction in the risk of clinical worsening events compared to placebo.
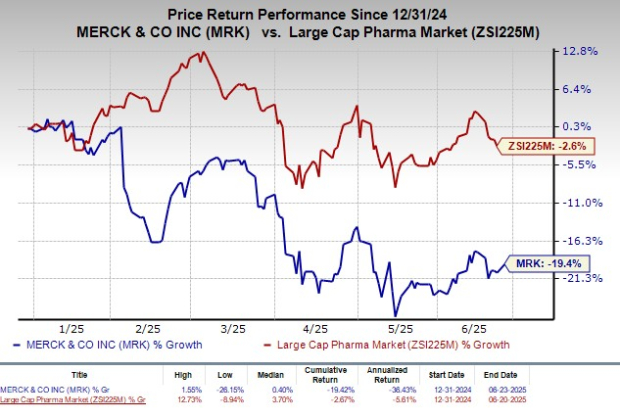
The HYPERION study was halted early due to overwhelming efficacy signals observed in previous studies. Year-to-date, Merck’s shares have fallen 19.4%, while the broader industry has decreased by 2.6%. Winrevair generated $280 million in sales in Q1 2025, a 40% increase sequentially.
Winrevair was FDA-approved in March 2024 and has the potential to significantly boost Merck’s revenue after the patent expiration of its blockbuster drug, Keytruda, in 2028.