Adagene Inc. (ADAG) has received Fast Track designation from the FDA for its lead candidate, muzastotug (ADG126), which is designed for adults with microsatellite-stable metastatic colorectal cancer (MSS mCRC) without active liver metastases. The designation was granted for its use in combination with Merck’s Keytruda (pembrolizumab).
This announcement led to a 13.8% increase in ADAG shares. The Fast Track status is aimed at expediting the development and review process for treatments addressing serious diseases with unmet needs. Muzastotug is currently in a phase II study with a planned phase III trial set for 2027.
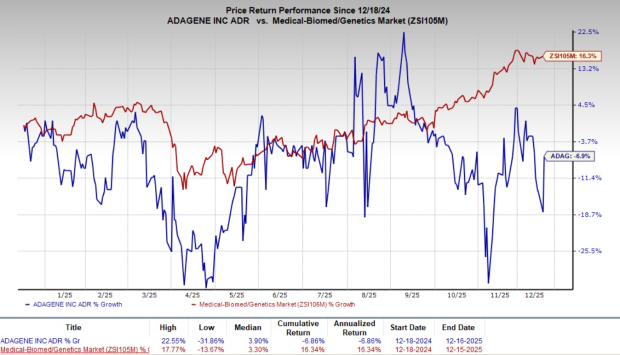
Over the past year, ADAG shares have decreased by 6.9%, while the industry average has risen by 16.3%.