bioAffinity Technologies Reports Strong Revenue Growth, Stock Soars
Shares of bioAffinity Technologies, Inc. (BIAF) have skyrocketed 160.9% following the company’s financial results for the year ending December 31, 2024. In contrast, the S&P 500 Index fell 8.9% during the same timeframe. Over the past month, BIAF stock achieved an impressive gain of 89.3%, compared to a 9.7% decrease in the S&P 500.
Find the latest EPS estimates and surprises on Zacks Earnings Calendar.
Record Revenue and Financial Overview
bioAffinity reported record revenues of $9.4 million for 2024, a significant increase of 269.7% from $2.5 million in 2023. This growth was mainly driven by a full year of operations from its acquired subsidiary, Precision Pathology Laboratory Services (PPLS). The company’s main product, CyPath Lung, experienced a remarkable 1,400% increase in orders compared to 2023, demonstrating a strong acceptance among healthcare providers.
Despite the revenue increase, the net loss expanded to $9 million, or $0.75 per share, compared to a loss of $7.9 million, or $0.91 per share, the previous year. Operating expenses climbed 74.3% to $18.3 million from $10.5 million, reflecting heightened commercial activities and the full-year impact of PPLS operations.
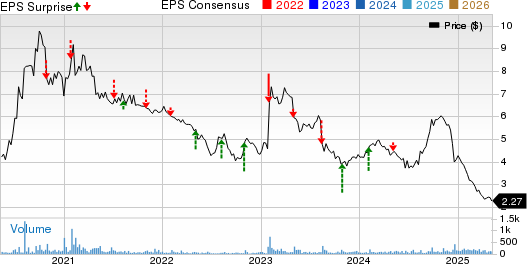
bioAffinity Technologies, Inc. Price, Consensus, and EPS Surprise
bioAffinity Technologies, Inc. price-consensus-eps-surprise-chart | bioAffinity Technologies, Inc. Quote
Key Business Metrics Analysis
The company’s selling, general, and administrative expenses rose 46.4% to $9.9 million from $6.8 million, largely due to increased personnel costs and commercialization efforts for CyPath Lung. Direct costs surged 243.7% to $5.9 million from $1.7 million, driven by the expansion of pathology and lab operations. R&D expenses remained stable at around $1.5 million, while clinical development costs rose by 25.3% to $0.32 million from $0.26 million. At the end of 2024, bioAffinity had $1.1 million in cash and equivalents, down from $2.8 million at the end of 2023. However, the company raised $1.4 million in February 2025 through warrant exercises, enhancing its liquidity.
Additionally, BIAF expanded its physician network by over 300% in 2024, setting a solid foundation for future growth.
Management Insights
Maria Zannes, President and CEO, noted that the record year validated the company’s growth strategy, particularly via the integration of Precision Pathology and the growing adoption of CyPath Lung. Zannes highlighted the test’s reimbursement by Medicare and private insurers, as well as its listing on the U.S. Federal Supply Schedule, ensuring access to 1,380 government healthcare facilities. These advancements, according to her, establish a basis for nationwide expansion beyond Texas. Moving forward, Zannes outlined the critical goals of scaling CyPath Lung, executing a pivotal FDA trial, and advancing the diagnostic pipeline utilizing AI and flow cytometry.
Driving Factors Behind Financial Performance
BIAF’s revenue expansion was primarily influenced by the full-year contributions from Precision Pathology and the increasing acceptance of CyPath Lung in the healthcare sector. An independent study reported that adding CyPath Lung to Medicare’s standard of care could have saved an average of $2,773 per patient in 2022, equating to total savings of $379 million. For privately insured patients, the savings were estimated at $6,460 per patient, totaling $895 million if all eligible individuals were treated.
Rising operating costs aligned with revenue growth, stemming from expansion-related expenses, including labor and overhead costs associated with Precision Pathology and intensified marketing efforts for CyPath Lung. Notably, both R&D and clinical development expenses remained constant year-over-year, though management expects an increase in clinical costs in 2025 due to the upcoming pivotal FDA study.
Future Guidance
For 2025, bioAffinity projections estimate revenues between $6 million and $8 million, including $1 million to $2 million from CyPath Lung sales. This anticipated decline from 2024 reflects the planned discontinuation of unprofitable pathology services.
Management believes that reductions in labor and overhead will help mitigate the impact of lost revenue, ultimately leading to improved profitability for the subsidiary laboratory. Furthermore, the company is bracing for higher clinical spending to support the pivotal trial for CyPath Lung.
Recent Developments
BIAF has submitted the pivotal study protocol to the Sterling Institutional Review Board, with plans to enroll approximately 3,500 patients and to initiate the trial in the second quarter of 2025. The company has also collaborated with Dr. Gordon Downie to publish patient case studies, highlighting CyPath Lung’s effectiveness in early cancer detection and preventing unnecessary invasive procedures.
On the international front, bioAffinity secured a Japanese patent for its proprietary method utilizing flow cytometry for lung disease detection, enhancing its intellectual property portfolio. The company also progressed in R&D aimed at diagnostics for chronic obstructive pulmonary disease and asthma, backed by recent international patent recognition in Japan.
Research Insights on Promising Stocks
From an extensive stock selection, 5 Zacks experts have identified their top pick, which is expected to rise over 100% in the coming months. Among these featured stocks, Director of Research Sheraz Mian has singled out one for its immense growth potential.
This highlighted company caters to millennial and Gen Z demographics, generating nearly $1 billion in revenue last quarter alone. After a recent pullback, now may be an ideal time for investors to consider this opportunity. While not all selections may succeed, this stock could outperform previous Zacks’ recommendations, such as Nano-X Imaging, which surged by +129.6% in under nine months.
Free: See Our Top Stock And 4 Runners Up
bioAffinity Technologies, Inc. (BIAF): Free Stock Analysis Report
This article originally published on Zacks Investment Research (zacks.com).
The views and opinions expressed herein are those of the author and do not necessarily reflect those of Nasdaq, Inc.