“`html
Viking Therapeutics (VKTX) is developing VK2735, a dual GLP-1 and GIP receptor agonist, for obesity treatment, currently undergoing clinical trials for its oral and subcutaneous formulations. The drug showed a 12.2% weight loss in patients at the highest dosage over 13 weeks, compared to 1.3% for placebo. However, associated dropout rates raised safety concerns.
Pfizer (PFE) announced a $4.9 billion acquisition of Metsera to enhance its obesity drug portfolio, marking its re-entry into the market after previous setbacks. This acquisition includes four clinical-stage programs and aims to increase competition with existing leaders in the sector, such as Eli Lilly (LLY) and Novo Nordisk (NVO), which are already generating significant revenue from obesity treatments. The U.S. obesity market is projected to reach $100 billion by 2030, intensifying competition.
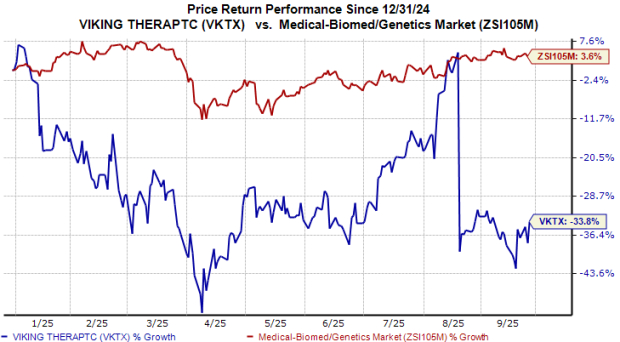
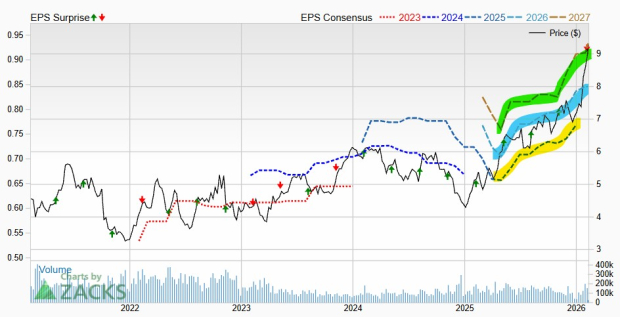
Despite recent challenges, Viking focuses on the subcutaneous version of VK2735, currently in late-stage development as its most viable option against the established competitors, with updates expected by early 2027. Viking’s share price reflects these pressures as it trades at a price-to-book value ratio of 3.76, higher than the industry average of 3.22.
“`