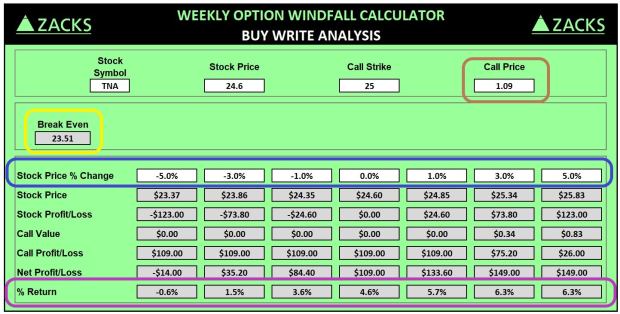
Cybin Inc., a clinical-stage biopharma company trading under the symbol CYBN, has revealed positive safety and efficacy results from its Phase 1 studies of CYB004 (intravenous administration) and SPL028 (intravenous and intramuscular administration) conducted on healthy volunteers.
Both compounds are innovative deuterated DMT molecules developed under the company’s next-generation psychedelics program, with a focus on addressing Generalized Anxiety Disorder (GAD.) DMT, a hallucinogenic tryptamine drug found in various plant species and animals, including humans, represents the foundation of these new molecules.
See Also: Cybin Officially Owns Small Pharma: Plans For New ‘International Leader’ In Psychedelic Therapeutics
Key Findings:
-
CYB004: This compound induced “robust” psychedelic effects surpassing the conventional dose-effect expectation, providing a more potent experience even at lower doses compared to DMT.
-
The psychedelic effects of CYB004 exhibited a rapid onset when administered intravenously over 5 minutes, lasting for approximately 40 minutes post-administration without requiring prolonged infusion.
-
CYB004 demonstrated good tolerability with minimal occurrence of serious adverse events, predominantly mild to moderate in nature and self-resolving.
-
SPL028, administered through intravenous and intramuscular routes, displayed both safety and good tolerability. Researchers identified a specific intramuscular dose eliciting a “breakthrough psychedelic experience,” ranging from 55 to 120 minutes for most individuals, typically leaning towards the shorter end of the spectrum.
Significance of the Results
The latest findings emphasize two drug profiles allowing for potential inter-data comparison. Both compounds exhibited concentrations within the “effective” range, with both intravenous and intramuscular routes demonstrating safety and good tolerability among participants.
Intramuscular (IM) dosing unveiled potent psychedelic effects with a short duration, hinting at a potentially “highly scalable” and “more convenient” dosing method in contrast to intravenous infusion.
Cybin’s CEO Doug Drysdale emphasized that these are “the first-in-human studies of deuterated DMT in healthy participants.” He expressed confidence in the highly promising intramuscular dosing results, which will aid in determining the dosing strategy for future clinical trials. Additionally, these findings are expected to save Cybin significant time and resources by negating the need for additional formulation studies for alternative methods, such as subcutaneous dosing.
The company anticipates that the comprehensive dataset on safety and tolerability from these studies will drive the commencement of a larger Phase 2 study involving patients with Generalized Anxiety Disorder in Q1 2024.
-
Photo: Benzinga edit with photo by aiyoshi597, Bacsica, Gisele Yashar and Gorodenkoff on Shutterstock.
Missed the initial surge in cannabis investments? Avoid repeating that miscalculation.
Experts are of the opinion that cannabis stocks have bottomed out and are now on the brink of unprecedented growth.
Join Benzinga PotProfits. Our in-house cannabis stock expert, Michael Berger, is committed to uncovering the most promising cannabis stocks poised for growth, even in a subdued market. He is leaving no stone unturned to bring you the most alluring potential double-digit opportunities!
Already this year, the PotProfits portfolio has recorded substantial gains such as:
- 47.10% with $GTBIF
- 40.23% with $TCNNF
- 21.50% with $VFF
But here’s the catch: Michael is on the verge of unveiling his next potential winners, and he’s itching to share these ticker symbols with you at the earliest opportunity.
Don’t miss out on the green rush!