EMA Approval Signals Hope for Fanconi Anemia Treatment
The European Medicines Agency (EMA) has given acceptance to Rocket Pharmaceuticals (RCKT) for their marketing authorization application (MAA) for RP-L102, a gene therapy designed to combat fanconi anemia (FA). This rare genetic disorder poses severe challenges to patients – targeting the bone marrow while increasing the risk of cancer development and congenital issues.
Scientific Breakthrough in Phase I/II Studies
RP-L102 showcased remarkable results in its phase I/II trial, demonstrating sustained genetic and phenotypic corrections alongside hematologic stabilization in FA patients. Moreover, the therapy exhibited exceptional tolerance among the study group.
New Hope on the Horizon
Rocket Pharmaceuticals disclosed intentions to pursue a similar filing for RP-L102 with the FDA based on the groundbreaking research. The company anticipates the submission to occur in the first half of 2024, ushering in a new era of treatment possibilities for FA patients.
Shifting Tides in Therapeutic Innovation
With its focus solely on developmental pipelines, Rocket depends on the success of its gene therapy initiatives for growth. The company’s dedication is evident in its range of potential treatments for cardiovascular and hematology conditions.
Expanding Horizons with Gene Therapy Portfolio
Besides RP-L102, Rocket is impending the PDUFA date of Jun 30 designated by the FDA for the BLA filing for Kresladi, a gene therapy targeting severe leukocyte adhesion deficiency-I (LAD-I), emphasizing a holistic approach to critical illnesses.
Shaping the Future of Healthcare
Looking beyond current advancements, Rocket is venturing into uncharted territories, exploring gene therapy candidates such as RP-L301 for pyruvate kinase deficiency (PKD) and RP-A501 for Danon disease (DD), showcasing the company’s commitment to pioneering medical breakthroughs.
Financial Implications and Market Position
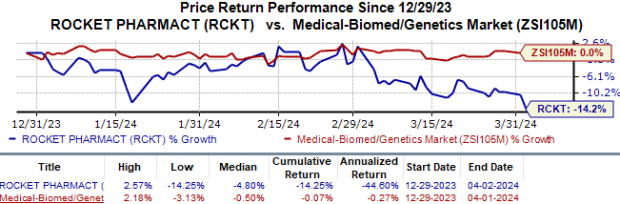
Rocket’s stock performance, reflecting a 14.3% decrease year-to-date, stands in stark contrast to the industry’s marginal 0.1% decline. However, with transformative therapies on the horizon, Rocket Pharmaceuticals is poised for a potential upsurge in the market.