“`html
GE HealthCare announced that its Revolution Vibe CT system has received FDA 510(k) clearance on September 2, 2023. This innovative imaging solution is designed for complex cardiac exams, addressing patients with arrhythmias, heavily calcified coronaries, and those with stents or bypasses.
The system is noted for its Unlimited One-Beat Cardiac imaging and AI-powered tools, leading to a 50% reduction in exam time and saving up to five minutes in patient preparation per scan. It also optimized CCTA exam scheduling and doubled CCTA capacity.
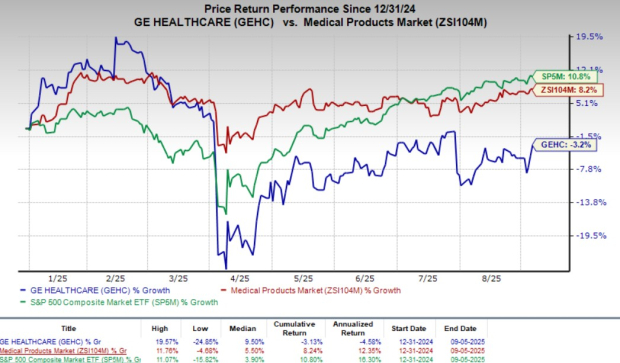
Cardiovascular disease is currently the leading cause of death globally, with projections of over 23 million deaths annually by 2030, highlighting an increasing demand for cardiac CT angiography. Following the announcement, GE HealthCare shares rose nearly 5.8%, although the company has experienced a 3.2% decline this year, while the industry gained 8.2%.
“`