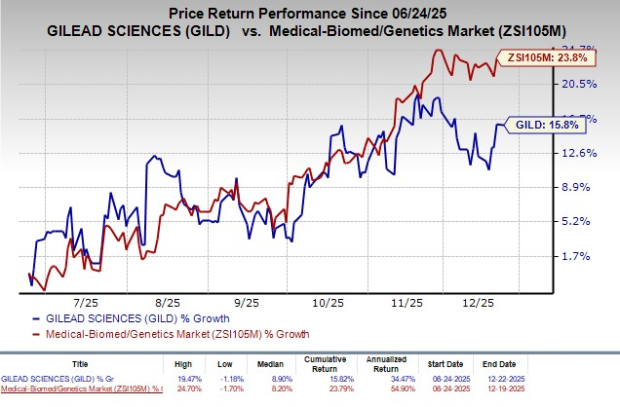
Gilead Sciences (GILD) has exercised its option to exclusively license Assembly Biosciences’ (ASMB) herpes simplex virus (HSV) helicase-primase inhibitor programs, including investigational candidates ABI-1179 and ABI-5366. This decision was made on October 5, 2023 as part of their ongoing collaboration and follows a 12-year partnership established in 2023. Gilead will pay Assembly Bio $35 million upfront and assume clinical development responsibilities for the HSV programs, which target the treatment of recurrent genital herpes impacting over four million people in the U.S. and parts of Europe.
Gilead is set to manage the clinical development and commercialization of these candidates, with Assembly Biosciences eligible for up to $330 million in milestone payments and tiered royalties based on net sales. Interim data from a phase Ib study indicated significant antiviral activity from these treatments, addressing a market where no new HSV therapies have been approved in over 25 years.