Gilead Sciences, Inc. (GILD) has received FDA approval for its injectable HIV-1 capsid inhibitor, lenacapavir, branded as Yeztugo, as a pre-exposure prophylaxis (PrEP) to prevent sexually acquired HIV in adults and adolescents weighing at least 35 kg. This makes Yeztugo the first and only twice-yearly PrEP option available in the U.S.
The FDA’s approval, based on data from late-stage studies PURPOSE 1 and PURPOSE 2, demonstrated that ≥99.9% of participants who received Yeztugo remained HIV-negative. This new treatment aims to overcome barriers to PrEP adoption, with the objective of improving HIV prevention among underserved populations.
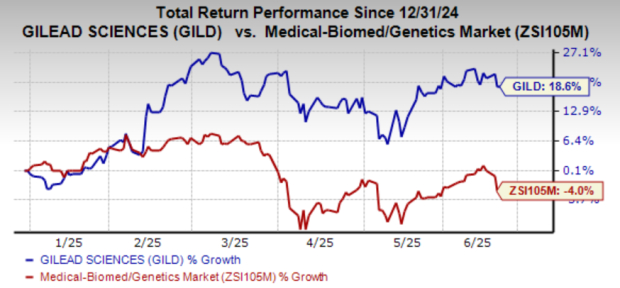
Gilead’s year-to-date stock performance shows an 18.6% gain, in contrast to a 4% decline in the industry. Yeztugo’s approval is poised to solidify Gilead’s position in the HIV treatment market, especially as its other prevention drug, Truvada, faces generic competition.