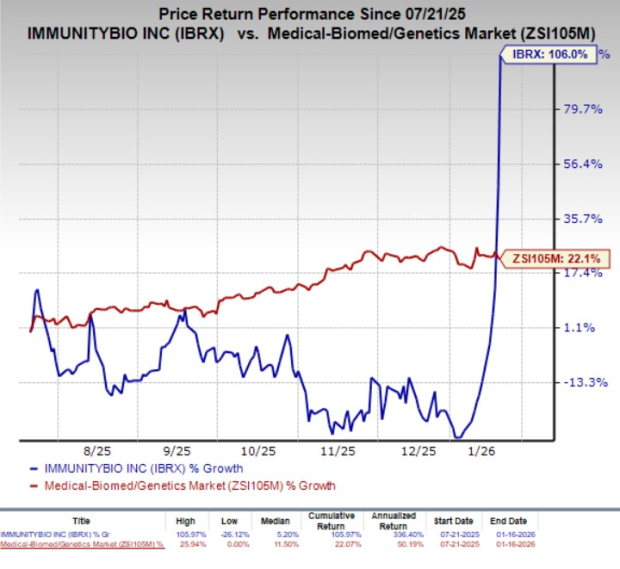
ImmunityBio (IBRX) experienced a significant stock surge, rising 39.8% on Friday and an additional 9.4% in after-hours trading, following the release of updated efficacy and safety data from its QUILT-106 clinical trial. The study, ongoing for patients with Waldenström non-Hodgkin’s lymphoma (NHL), evaluates the combined effectiveness of ImmunityBio’s allogeneic CD19 CAR-NK therapy and Roche’s Rituxan (rituximab). The updated data shows a 100% disease control rate, with durable complete responses extending up to 15 months.
In the trial, four patients have been enrolled, all of whom remain under clinical disease control, with two patients showcasing complete remission at seven and 15 months, respectively. The treatment approach, administered entirely on an outpatient basis without chemotherapy-based lymphodepletion, highlights ImmunityBio’s novel CAR-NK therapy as a potential alternative to conventional CAR-T therapies, which often require inpatient hospitalization.
Additionally, enrollment in ImmunityBio’s first-line BCG-naïve non-muscle invasive bladder cancer (NMIBC) study has exceeded expectations, with over 85% of the planned patient population enrolled. The company aims for full enrollment by Q2 2026 and plans to submit a biologics license application to the FDA by late 2026.