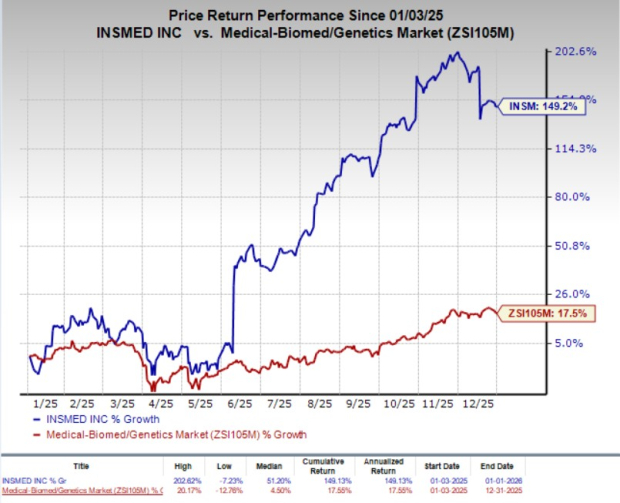
**Insmed’s shares (INSM) have soared 78% over the past six months following the FDA approval of brensocatib, marketed as ‘Brinsupri’, in August 2025. This marked the first approved therapy for non-cystic fibrosis bronchiectasis (NCFB). The drug generated $28.1 million in revenue during the third quarter, indicating strong market demand. Insmed’s stock has outperformed its industry, experiencing a 149.2% increase over the past year compared to 17.5% industry growth.**
**In addition to Brinsupri, Insmed’s established drug Arikayce has reported significant sales of $314.5 million for the first nine months of 2025, demonstrating a 21% increase year-over-year. The company has raised its guidance for Arikayce’s full-year revenues to between $420 million and $430 million. Despite a setback in a recent study of brensocatib for chronic rhinosinusitis, Insmed is expanding its pipeline with new treatments expected to be evaluated in 2026.**