Ionis Pharma, a leader in RNA-targeted therapeutics, has proudly announced that the FDA has bestowed its orphan drug designation upon Olezarsen for the treatment of familial chylomicronemia syndrome (FCS), a rare genetic disease that afflicts a minute segment of the populace.
Rare Recognition
The orphan drug designation, an accolade reserved for medicines catering to rare diseases affecting fewer than 200,000 individuals in the United States, underscores the unique potential of Olezarsen in addressing this challenging condition.
Proven Efficacy
In a landmark achievement for Ionis, the pipeline candidate Olezarsen, having already secured FDA’s Fast Track designation for FCS treatment, boasted positive data from the phase III BALANCE study. The conclusive data illuminated the substantial reduction in acute pancreatitis attacks and significant decrease in triglyceride levels, affirming the therapeutic prowess of Olezarsen.
Gauging Optimism
Emboldened by Olezarsen’s favorable clinical profile in FCS treatment, Ionis plans to file a new drug application (NDA) with the FDA based on the encouraging results from the BALANCE study. A potential approval for Olezarsen would mark a significant milestone as Ionis’ inaugural independently launched medicine, reflecting its commitment to addressing unmet medical needs in the rare disease arena.
Diversified Pipeline
Buoyed by the progress of Olezarsen, Ionis continues to fortify its wholly owned pipeline with other promising candidates such as ulefnersen and donidalorsen, both in advanced developmental stages. Equipped with a robust lineup, Ionis is primed to reshape the therapeutic landscape for an array of complex diseases.
Strategic Partnerships
Ionis’ strategic collaborations with industry titans including Biogen, AstraZeneca, Novartis, and GSK have borne fruit, exemplified by the commercial success of Spinraza and the recent FDA approval of Wainua for treating hereditary transthyretin-mediated amyloid polyneuropathy.
Market Potential
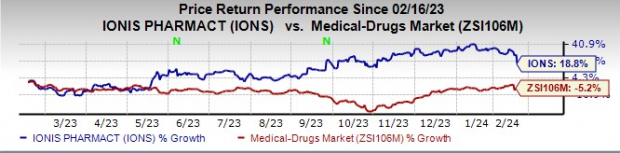
The market potential for Ionis’ diversified pipeline, bolstered by strategic partnerships and its independent endeavors, has resonated well with investors, as evidenced by the steady rise in Ionis’ stock price in the past year.
Promising Future
With a robust R&D engine and a promising portfolio, Ionis Pharmaceuticals is well-poised to significantly impact the rare disease landscape and deliver substantial value to patients and investors alike.