Gilead Sciences, Inc. (GILD) has received FDA approval for its twice-yearly injectable HIV prevention therapy, lenacapavir, marketed as Yeztugo, marking the first such option available in the United States. This follows a significant boost to GILD’s HIV portfolio which has been driven by the sales of flagship therapies such as Biktarvy and Descovy. The approval is particularly timely, as GILD faces increased generic competition with its existing prevention drug, Truvada.
In addition to lenacapavir, Gilead recently reported positive results from the phase III ARTISTRY-2 study for an investigational single-tablet HIV treatment regimen combining bictegravir and lenacapavir. This regimen met its primary endpoint for non-inferiority to Biktarvy and further strengthens GILD’s position in the HIV treatment market. Gilead plans to submit these findings to regulatory authorities, enhancing the likelihood of approval.
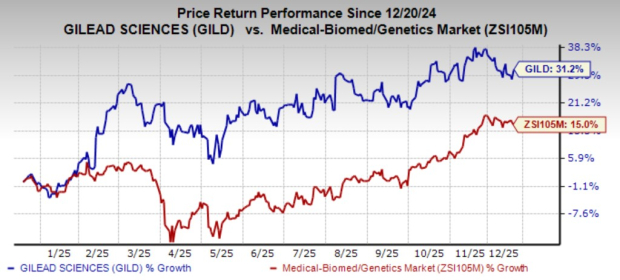
Gilead’s stock has increased by 31.2% over the past year, while competitors like GSK plc (GSK) and Merck & Co., Inc. (MRK) continue to grow their own HIV treatments amidst fierce competition in the sector.