MAIA Biotechnology’s Breakthrough in Oncology
MAIA Biotechnology (NYSE:MAIA) entered the biotech IPO scene in 2022 with a modest $20 million market cap. The company’s recent release of promising preliminary Phase 2 data in NSCLC has set the stage for a potential game-changer in the treatment of solid tumors. The lead asset, THIO, boasts a unique mechanism that targets and obliterates telomeres to selectively eliminate cancer cells. Despite the groundbreaking potential, the broader market seems oblivious to the asymmetric investment opportunity that MAIA presents. As the company prepares to demonstrate the profound and enduring efficacy of its treatment in 2024, a goldmine may await investors who recognize its worth.
In September 2023, the most recent data cutoff revealed that THIO’s disease control rates (DCRs) of approximately 90% — encompassing patients with tumor shrinkage or stable disease (SD) — outperformed the standard of care (SoC) for both second- and third-line NSCLC patients. Additionally, the overall response rates (ORRs) for THIO’s registrational dose of 180 mg are projected to reach over 30%. MAIA is set to provide a data update at the JPM Healthcare Conference in the upcoming week and plans to pursue accelerated approval in NSCLC in 2025 pending continued efficacy.
THIO, administered in combination with an immune checkpoint inhibitor (ICI), has the unique ability to activate the innate and adaptive immune system. The ongoing Phase 2 trial involves the use of THIO in combination with Regeneron’s Libtayo, for which MAIA holds a clinical supply agreement in NSCLC. Another potential upcoming catalyst is a partnership with another major pharma player in the oncology space, possibly in one of THIO’s three orphan drug designated (ODD) indications.
THIO, with its elegant mechanism and an incredibly benign safety profile for an oncology treatment, shows preliminary signs of superiority over the current standard of care. The future looks bright for MAIA, with the potential of its treatment becoming the new standard of care in first-line refractory NSCLC patients, and further possibilities in other solid tumor types and earlier lines of therapy. With a market cap of only around $20 million, MAIA certainly offers a significant speculative position in the field of oncology therapy.
The Scientific Story Behind THIO
THIO (6-thio-dG) underwent initial development and research at the NIH in the 1970s as a chemotherapy treatment for various tumor types, albeit at significantly higher doses than the ones MAIA is currently testing. Despite early setbacks, the compound regained attention when scientific knowledge of telomeres took a leap in the 1990s and early 2000s. In the mid-2010s, THIO was reintroduced to research by Dr. Jerry Shay’s lab at UT Southwestern. Dr. Ilgen Mender, a researcher at Dr. Shay’s lab, published a seminal paper elucidating THIO’s mechanism of action in Cancer Cell in 2020.
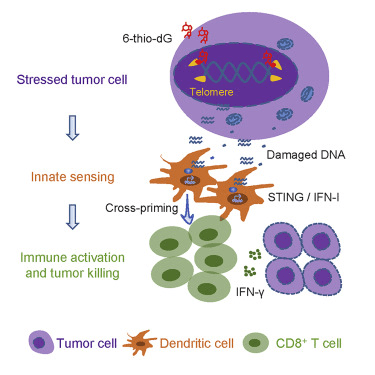
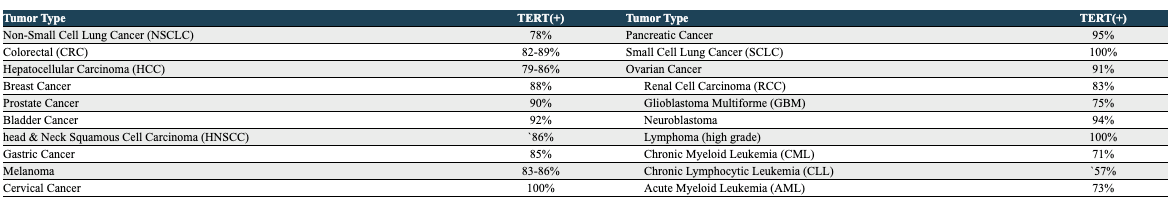
THIO, a nucleoside analog, becomes part of telomeres via the enzyme telomerase. Telomeres, the protective “caps” on DNA ends, are built and maintained by telomerase, primarily expressed during early growth and development and in select cell types in adulthood. However, in most cancers, telomerase expression is reactivated, leading to a state of “replicative immortality,” enabling uncontrolled and indefinite cell division. Notably, telomerase has been found to be expressed in over 80% of NSCLC tumors.
As a nucleoside analog, THIO directly incorporates into the telomere via telomerase, causing instability and unraveling of the DNA, ultimately leading to cell death. Dr. Mender’s 2020 paper also revealed THIO’s immunogenic effect, where the damage to telomerase-expressing cells triggers the innate immune system, resulting in an adaptive (T cell) immune response. This unique property allows THIO to sensitize the immune system to immunologically “cold” tumors and overcome PD-L1 (checkpoint) inhibitor resistance.
THIO’s mechanism differs from Geron’s lead candidate, imetelstat, a telomerase inhibitor. THIO’s mechanism is superior as it actively eliminates telomerase-expressing cells, in contrast to inhibiting telomerase, which allows the cell to divide until the existing telomere runs out. MAIA’s Chief Scientific Officer, Dr. Sergei Gryaznov, a world-renowned leader in telomeres and the co-inventor of imetelstat, made the strategic move to MAIA after being rejected by Geron regarding the pursuit of THIO.
Exceptional Preclinical Results
THIO, through research conducted by MAIA and Dr. Shay’s lab, has displayed impressive preclinical results across various solid tumor types and in combination with multiple ICIs, such as Libtayo, Keytruda, and Tecentriq. While an in-depth analysis of THIO’s potential in non-NSCLC is reserved for a future article, its remarkable effects in colorectal cancer and hepatocellular carcinoma (HCC) deserve emphasis. In mouse models representing both diseases, THIO achieved a 100% complete response rate, signifying a complete eradication of the tumors.
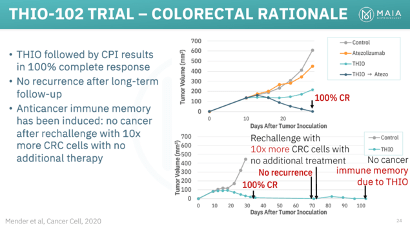
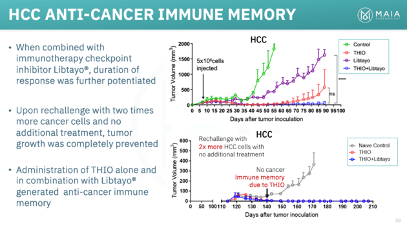
THIO’s selective activity in telomerase-expressing cells has enabled an exceptionally safe profile, particularly within the field of oncology. This safety has led to FDA Orphan Drug Designation for THIO in HCC, small cell lung cancer (SCLC), and more recently, glioblastoma.
Moreover, the curative dose of THIO in mice was observed at 60 mg per cycle, as opposed to the established maximum tolerated dose in humans of 2,500 mg per cycle, demonstrating an extensive therapeutic margin.
MAIA’s THIO-101: Transforming NSCLC Treatment Landscape
Regeneron Collaboration and Promising Prospects
Following the emergence of THIO-101’s Phase 2 trial results, MAIA successfully secured a clinical supply deal with Regeneron, a strategic move that holds the potential to significantly impact the non-small cell lung cancer (NSCLC) treatment landscape. With the estimated worth of the Regeneron agreement pegged at a substantial $32 million, the collaboration highlights the growing interest and confidence in THIO’s capabilities.
Shaping the NSCLC Treatment Paradigm
NSCLC, considered the largest cancer type globally by sales, presents a formidable battleground in the quest for effective therapies. With approximately 140,000 NSCL patients in the US, a significant portion of whom possess an addressable genetic mutation such as EGFR or KRAS, the demand for innovative treatment options is palpable. THIO’s focus on non-genetic patients, for whom existing treatments yield suboptimal benefits, underscores the pressing need for breakthrough advancements in NSCLC treatment.
Pioneering Pathways in Treatment Resistance
As a potential second- and third-line treatment option, THIO is poised to challenge the existing status quo. The prevailing dearth of clear-cut standard care in these therapeutic stages sets the stage for THIO’s innovative potential to fill crucial gaps. With third-line patients facing particularly bleak prognoses and limited treatment options, the emergence of THIO carries the promise of a much-needed paradigm shift.
Setting Ambitious Regulatory Targets
Driven by the urgency of addressing critical clinical needs in NSCLC, MAIA is actively pursuing accelerated approval for THIO. With ambitious targets for Overall Response Rate (ORR) and Duration of Response (DoR) set as benchmarks for accelerated approval, the company is charting a bold course to catalyze pivotal regulatory advancements in the NSCLC treatment landscape.
THIO-101: Charting a Promising Trajectory
MAIA’s THIO-101 heralds a potential breakthrough in the treatment of NSCLC. With a focus on second- and third-line patients who confront limited treatment options, THIO’s evolving data presents a beacon of hope for a patient cohort that has long been underserved by conventional therapies.
Groundbreaking Data Analysis of THIO’s Early-stage Preliminary Signs
THIO, weary from the relentless dance of early-stage investment, has ushered in a new era of optimism for the medical community. The preliminary data from the MAIA program, though modest, bears the promising beacon of hope, standing as a testament to perseverance, innovation, and relentless pursuit of advanced medical solutions.
Preliminary Evaluation
As of the data cutoff, 49 patients have been enrolled in the MAIA program, with 37 patients undergoing at least one follow-up scan. This initial foray into the uncharted waters of medical discovery has produced encouraging early signs for THIO. While the data is in the early stages and requires contextual interpretation, the foothold established by these initial findings is an encouraging portent.
Patient Outcomes
Of the 37 patients evaluated, 33 remain alive, marking a commendable survival rate, especially considering the challenging landscape of treating late-stage non-small cell lung cancer (NSCLC). The tenacity displayed by the pilot patients, particularly the third-line patients from Australia who achieved survival periods of 12.5 and 14 months, is nothing short of extraordinary. The subsequent patients enrolled in Europe, spanning 1-6 months of treatment, have also demonstrated promising signs.
Disease Control and Treatment Response
The overall Disease Control Rate (DCR) stands at an impressive 92%, with a breakdown of 100% for second-line patients and 88% for third-line and beyond patients. The ability of the treatment to maintain disease control in 73% of patients as of the data cutoff is an encouraging sign for THIO’s potential impact in the field of NSCLC therapy.
The level of partial and complete responses, though appearing somewhat modest at first glance, hints at potential undercurrents of promise. While only 15% and 0% of patients reported partial and complete responses respectively, the early stage and the confirmation that the 180 mg dose is achieving an over 35% Objective Response Rate (ORR) provide a glimmer of optimism for the future.
Second-Line Patient Breakdown
In the second-line cohort, where 17 patients were enrolled, the initial DCR stood at a noteworthy 100%. While unfortunate, disease progression was experienced by three patients in their latest scans. The fact that these patients could potentially regain stable disease status presents an intriguing avenue for further exploration.
Third-Line+ Analysis
The data from the 20 third-line+ patients revealed an 88% DCR and four partial responses, indicating a promising trajectory. These findings establish THIO’s 20% partial response rate as approaching the level set by standard second-line therapies, hinting at a potential paradigm shift in the field.
Potential 180 mg Dose Advancements
The selection of the 180 mg dose for potential accelerated approval carries significant weight, especially considering the reported 35%+ ORR achieved at this dosage. The implications of this data, in terms of patient responses and subsequent progress, are intriguing and warrant close observation.
The company has announced plans to enroll 100-120 patients in the 180 mg dose cohort, marking a crucial stage in THIO’s developmental journey.
Further Insight
As the medical community continues to dissect and interpret the data, it is important to consider the broader implications of THIO’s efficacy profile. The initial Disease Control Rate of 100% and an ongoing rate of 70% for second-line patients paints an encouraging picture, especially when compared to existing therapies.
- Comparatively, THIO’s DCR surpasses current standards of care (SoC) by a significant margin, marking a potential watershed moment in NSCLC treatment.
- Moreover, the 20% ORR in the third-line cohort displays potential to rival established second-line therapies, signaling a potential inflection point in the progression of NSCLC treatment.
- The success with the 180 mg dose and the atypical survival periods of the early patients are encouraging markers for THIO’s future.
Potential for Delayed Responses
The intriguing anecdote of the first patient enrolled in THIO-101 (Patient 01-003) underscores the potential for delayed treatment responses, further adding depth to the ongoing analysis of THIO’s impact. The patient’s perseverance and the stability of their disease status are emblematic of the potential endurance offered by THIO’s therapeutic approach.
The Promise of THIO + Libtayo in Transforming Oncology Treatment
As THIO + Libtayo continues to progress, it delivers a message of hope to cancer patients and investors alike. The potential of this innovative treatment to revolutionize oncology stands as a beacon of light in the medical landscape. Through a deeper exploration of the recent developments and upcoming milestones, it becomes clear that THIO + Libtayo is on the cusp of making a significant impact.
The Momentum of Progress
The recent clinical data showcases powerful examples of the sustained effectiveness of THIO + Libtayo. Notably, the encouraging case of a patient reporting 28% tumor shrinkage from baseline, even months after treatment cessation, signifies the burgeoning potential of this treatment beyond conventional timelines.
Furthermore, the delayed effect of THIO + Libtayo, as seen in patients achieving responses in subsequent scans after initial disease control, underscores its unique ability to activate and entrain the immune system. These instances resonate as a testament to the enduring impact of this treatment regimen.
It is worth noting that such observations align with known phenomena related to immune checkpoint inhibitors, providing a compelling foundation for the far-reaching implications of THIO + Libtayo in the realm of oncology.
Outlook and Prospects
Looking ahead, the upcoming data update holds significant promise for THIO’s clinical profile. With an anticipated influx of new patient data and post-baseline scans, the narrative surrounding THIO + Libtayo is poised to gain further depth and validation.
Crucial questions pertaining to the deepening of patient responses, resilience in the face of progression, and the trajectory of survival will soon find answers, shedding light on the enduring impact of this treatment.
If the responses to these questions lean in a favorable direction, the stage will be set for THIO + Libtayo to carve a distinctive niche in the field of oncology, marking a paradigm shift in the approach to cancer treatment.
Navigating the Regulatory Landscape
DCR vs ORR
While second-line ORR remains a feasible endpoint for accelerated approval, the pronounced divergence in DCR poses a compelling case for the robustness of THIO’s efficacy. This metric’s established correlation with overall survival underscores the potential of THIO + Libtayo to fundamentally influence patient outcomes.
Second-Line vs. Third-Line
The ongoing success of THIO in both second- and third-line treatments accentuates the optionality for accelerated approval. This distinctiveness in performance vis-à-vis standard-of-care therapies highlights the transformative impact that THIO + Libtayo could engender in the oncological domain.
Regular Approval and Investigator-Initiated Trials
The regulatory pathway for THIO remains multifaceted, affording avenues for both accelerated and traditional approvals. Moreover, the potential for investigator-initiated trials stands to further bolster the approval dossier, accentuating the far-reaching implications of this treatment regimen.
“Homerun” Scenario and Accelerated Approval
With the progression of clinical data and the projection of enduring responses, the envisaged scenario of substantial investment and scientific interest underscores the transformative potential of THIO + Libtayo. Such a prospect could punctuate a pivotal juncture in the oncological landscape.
In summary, the horizon for THIO + Libtayo appears resplendent with the promise of breakthroughs that could redefine the paradigms of oncology treatment, offering renewed hope to patients and investors alike.
MAIA’s Groundbreaking Oncology Breakthrough Set to Rock the Solid Tumor Treatment Landscape
MAIA is on the verge of a revolutionary breakthrough in the treatment of solid tumors, particularly non-small cell lung cancer. The company’s flagship drug, THIO-101, has shown promising potential, as demonstrated by its granting of three ODDs.
Clinical Breakthrough
MAIA’s groundbreaking drug, THIO-101, has demonstrated significant potential in the treatment of solid tumors, particularly non-small cell lung cancer. The drug has received three Orphan Drug Designations, indicating its potential to address critical unmet medical needs in cancer treatment.
MAIA has plans to initiate a basket trial in colorectal and other solid tumors, which is indicative of their confidence in the broad-spectrum oncology therapy potential of THIO-101. The company envisions a future where their pipeline includes more potent telomere-targeting agents similar to THIO-101, further expanding their impact in the oncology space.
Strategic Partnerships and Funding
As MAIA gears up to report data from 50-60+ patients in the coming weeks and months, the potential for strategic partnerships with industry giants such as Regeneron and other leading pharmaceutical companies looms large. The company’s partnership with Regeneron, in particular, could alleviate significant funding burdens in the near term, providing a much-needed financial boost.
Furthermore, with prospective partners like Roche/Genentech and Merck in the picture, MAIA stands to solidify its position as a key player in the oncology space through strategic collaborations.
Market Valuation
The potential market valuation for MAIA’s THIO-101 is staggering, with just a 10% penetration of the 140k US NSCLC market projected to yield $1.4 billion in sales. The company’s value proposition is further reinforced by the success of comparable companies such as Mirati, which was acquired for $4.8 billion, highlighting the immense valuation potential for MAIA.
The company’s extensive upside potential, even in a modest micro-cap status, signifies a massive 5-10x opportunity. As MAIA continues to make strides in the oncology landscape, the potential for a multi-billion-dollar impact on solid tumor treatment cannot be understated.
Navigating Risks
-
As with any clinical venture, MAIA faces inherent risks in the development of THIO-101, particularly in the face of patient fatalities and the need for extended data to validate the drug’s efficacy over time.
-
The company’s reliance on data maturation and response deepening further compounds the risks, as does the departure from preclinical expectations, necessitating a cautious approach to mitigate potential setbacks.
-
Operational shifts within the company, such as changes in key leadership positions, serve as additional nuances that require astute management to navigate effectively amid the potential headwinds.
Path to Progress
MAIA’s journey towards establishing THIO-101 as a groundbreaking oncology therapy represents an exciting inflection point in the field of cancer treatment. As the company continues to forge ahead with their clinical readouts over the next 6 months, the potential for a paradigm shift in solid tumor treatment looms large on the horizon.
While inherent risks persist, the emergence of THIO-101 as a durable and potent treatment option could herald a new era of hope for cancer patients and physicians alike, positioning MAIA at the vanguard of innovation in the oncology space.
Editor’s Note: This article covers one or more microcap stocks. Please be aware of the risks associated with these stocks.