Shares of Moderna, Inc. MRNA were down 4.4% on May 10 after the company announced that the FDA has delayed the review timeline for its mRNA-based, respiratory syncytial virus (RSV) vaccine, mRNA-1345.
Moderna has developed mRNA-1345 for use in older adults (aged 60 years and above).
The regulatory body notified the company that due to administrative constraints, it would not be able to complete the review of the biologics license application (BLA) for mRNA-1345 by the Prescription Drug User Fee Act date of May 12, 2024.
The FDA has extended the review time for the above BLA by a couple of weeks. The review is now expected to be completed by the end of this month.
The FDA has, however, not identified any issues related to the safety, efficacy or quality of mRNA-1345, which could have delayed the approval of the vaccine.
Moderna believes that the extended review timeline for mRNA-1345 is not likely to affect the launch plans for the vaccine. The Centers for Disease Control and Prevention (“CDC”) Advisory Committee on Immunization Practices is expected to review mRNA-1345 in its meeting to be held in June 2024, which is a necessity before the commercial launch of the vaccine.
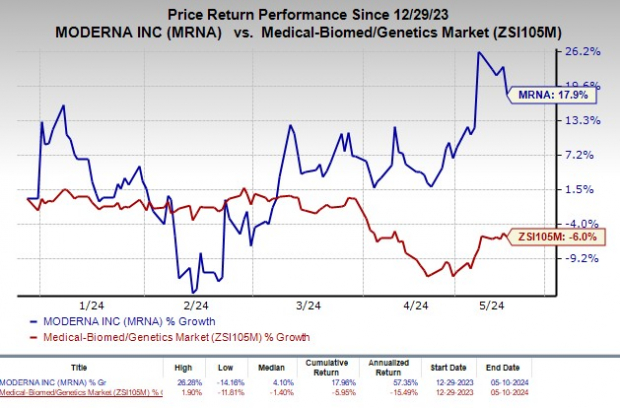
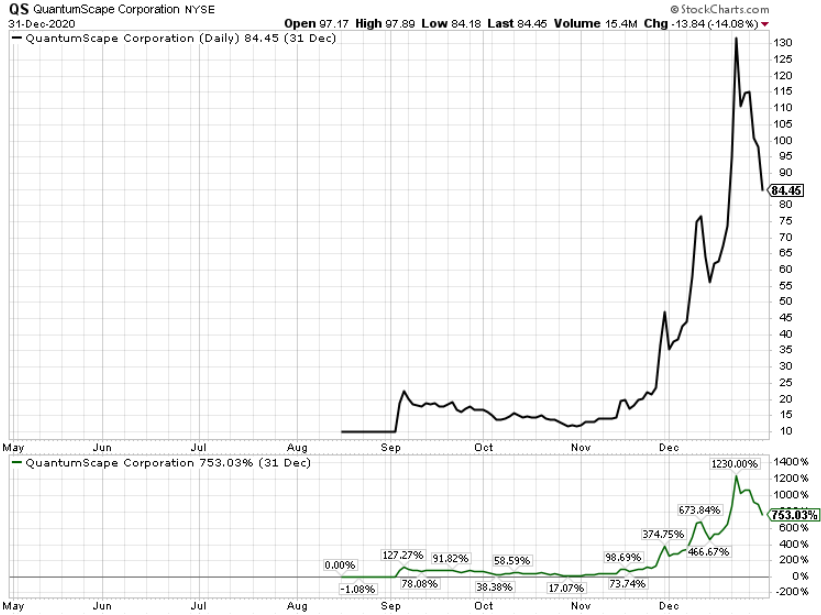
Shares of Moderna have rallied 17.9% so far this year against the industry’s decline of 6%.

Image Source: Zacks Investment Research
Moderna initiated regulatory submissions for mRNA-1345 in several markets, including the United States and Europe, in July 2023. The regulatory filings are based on positive data from an interim analysis of the pivotal phase III ConquerRSV study. Data from the study showed that participants who received mRNA-1345 achieved vaccine efficacy of 83.7% against RSV-associated lower respiratory tract disease (RSV-LRTD), as defined by two or more symptoms of the disease.
If approved, mRNA-1345 could be Moderna’s second product to market, whose commercial launch is expected in the third quarter of 2024. Moderna is also working on expanding the use of mRNA-1345 in pediatric populations.
The delay in the review of mRNA-1345 puts it at a competitive disadvantage to GSK GSK and Pfizer PFE, which have already launched their respective RSV vaccines, Arexvy and Abrysvo, to prevent RSV-LRTD in older adults in the United States.
The CDC has also recommended the use of both GSK and Pfizer’s RSV vaccines for older adults.
GSK’s Arexvy witnessed an exceptional launch activity and generated £1.2 billion in sales in 2023. Vaccine sales are expected to be even stronger in 2024, driven by further penetration in the United States as well as early adoption in international markets. Arexvy generated sales worth £182 million in the first quarter of 2024.
PFE’s Abrysvo also witnessed a strong uptake as the vaccine generated $890 million in sales in 2023. The strong momentum is expected to drive sales further in 2024. Abrysvo is also approved for use in infants through maternal immunization. Abrysvo generated sales worth $145 million in the first quarter of 2024.
Earlier this month, along with its earnings release, Moderna reiterated its product revenue guidance for 2024. It expects to generate around $4 billion in product revenues, driven mainly by the sales of its COVID-19 vaccine and the launch of its RSV vaccine.
Zacks Rank & Stock to Consider
Moderna currently has a Zacks Rank #3 (Hold).
A top-ranked stock in the healthcare sector is Ligand Pharmaceuticals Incorporated LGND, sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Ligand’s 2024 earnings per share have improved from $4.42 to $4.56. Year to date, shares of LGND have risen 22.2%.
Earnings of LGND beat estimates in each of the trailing four quarters, the average surprise being 56.02%.
Buy 5 Stocks BEFORE Election Day
Biden or Trump? Zacks is releasing a FREE Special Report, Profit from the 2024 Presidential Election (no matter who wins).
Since 1950, presidential election years have been strong for the market. This report names 5 timely stocks to ride the wave of electoral excitement.
They include a medical manufacturer that gained +11,000% in the last 15 years… a rental company absolutely crushing its sector… an energy powerhouse planning to grow its already large dividend by 25%… an aerospace and defense standout that just landed a potentially $80 billion contract… and a giant chipmaker building huge plants in the U.S.
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
To read this article on Zacks.com click here.
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.