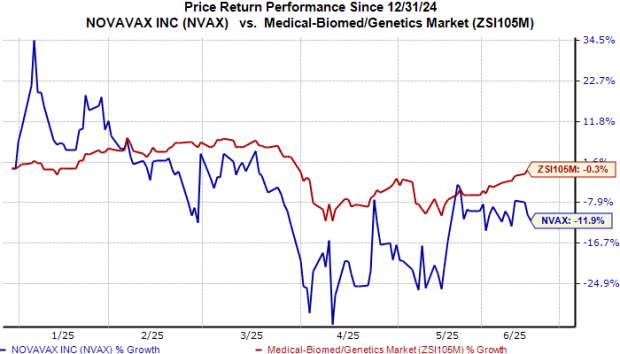
Novavax (NVAX) announced positive results from an initial cohort of a late-stage study evaluating its COVID-19-influenza combination (CIC) and stand-alone influenza vaccines in adults aged 65 and older. The study showed that both vaccines produced robust immune responses against three flu strains (H1N1, H3N2, and B) and the SARS-CoV-2 strain, comparable to Novavax’s approved COVID-19 vaccine, Nuvaxovid, and Sanofi’s Fluzone HD.
While the study aimed to provide preliminary immunogenicity data rather than statistical significance, Novavax plans to use these findings for another late-stage study potentially supporting regulatory submissions. The announcement follows Novavax’s recently received FDA approval for Nuvaxovid for older adults, though with restrictions for younger individuals with underlying health conditions. Additionally, Sanofi has acquired global marketing rights for Nuvaxovid, barring certain territories.
Novavax is also exploring strategic collaborations for further development of its vaccines. Competitors like Pfizer and Moderna are also working on their own COVID-flu combination vaccines, with Moderna’s candidate, mRNA-1083, facing additional data requirements before resubmission to the FDA.