FDA’s New Regulations Spark Rally in AI-Enabled Biotech Stocks
The U.S. Food and Drug Administration (FDA) has announced significant changes to animal testing requirements for new drugs, leading to a surge in the stock prices of AI-enhanced biotech companies like Recursion Pharmaceuticals Inc. RXRX and Ginkgo Bioworks Holdings Inc. DNA last week.
Details of the Announcement
On Thursday, Martin Makary, the new FDA commissioner, revealed reforms regarding drug evaluation standards. According to the agency’s press release, the requirement for mandatory animal testing will be eliminated for monoclonal antibody therapies and other drugs. Instead, the FDA will introduce “more effective, human relevant methods.”
The FDA’s approach includes various alternatives to animal testing, such as AI-based computational models of toxicity, cell cultures, and organoid toxicity testing conducted in lab environments.
More Coverage: FDA Approves Bayer’s Drug for Specific Cancer Mutation
Reactions from the Industry
Commissioner Makary described the decision as a “win-win for public health and ethics,” arguing that drug manufacturers had unnecessarily prolonged animal testing for many drugs already established in human use across various markets.
This policy change contributed to significant stock price increases for AI-centered companies: Recursion Pharmaceuticals rose by 27.72%, Ginkgo Bioworks increased by 11.23%, Absci Corp. ABSI by 23%, and Schrodinger Inc. SDGR by 27.42% following the announcement.
Conversely, companies involved in the preclinical laboratory services sector, like Charles River Laboratories Inc. CRL and Inotiv Inc. NOTV, saw their shares decline sharply—down nearly 28% and 44%, respectively, as they are recognized legacy players in the contract research organization (CRO) industry.
Analyst Perspectives
Biotech analysts have offered varied opinions regarding the FDA’s announcement. Analysts at Leerink Partners labeled the move as “more public relations than an actual policy shift,” forecasting limited immediate effects on preclinical testing practices, according to reports from BioSpace.
On a more optimistic note, analyst Rick Weissenstein from TD Cowen described the initiative to Axios as blending deregulatory efforts with advanced technology to support a popular objective: reducing animal testing.
Weissenstein further mentioned that this FDA initiative could streamline drug development by leveraging AI and computational models, leading to cost reductions and improved patient safety.
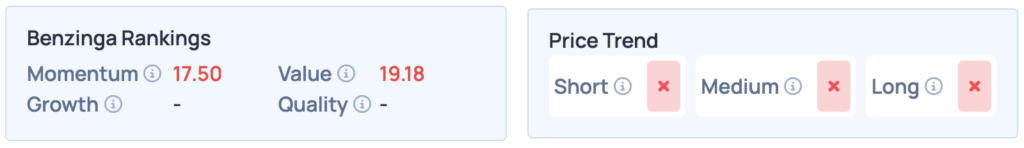
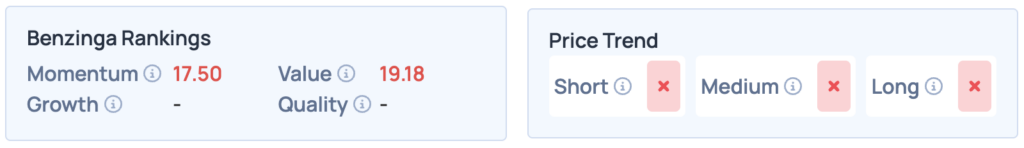
Despite the recent stock surge, Recursion Pharmaceuticals ranks lower on Benzinga’s Edge Stock Rankings. Questions remain for Ginkgo Bioworks and Schrodinger regarding their positions. For greater insights, consider signing up for Benzinga Edge Stock Rankings.
Read More: Insights on Whales Betting on Recursion Pharmaceuticals
Photo courtesy: Shutterstock
Market News and Data brought to you by Benzinga APIs