On February 16, Sarepta (SRPT) revealed that the FDA has accepted its efficacy supplement to the biologics license application (BLA) for Elevidys, a treatment of Duchenne muscular dystrophy (DMD). This ground-breaking development has catapulted the company’s stock by 7.8%, marking a momentous stride for both Sarepta and the DMD treatment landscape.
FDA Priority Review
The FDA has granted the Elevidys filing a Priority Review status, indicative of accelerated regulatory action, potentially reducing the review period by four months. Investors have their sights set on an eagerly anticipated final decision from the FDA, expected on Jun 21, 2024.
Historical Context
Last year, Elevidys was granted FDA approval under the accelerated pathway to treat ambulatory pediatric patients aged between four and five years with DMD. This landmark decision positioned Elevidys as the first approved gene therapy for DMD, setting a monumental precedent for the future of DMD management.
The Role of Efficacy Supplement
The efficacy supplement aims to broaden Elevidys’ labeled indication to encompass all DMD patients, regardless of age and ambulatory status. Furthermore, it endeavors to transition Elevidys’ accelerated approval to a traditional approval, indicating potential for broader therapeutic impact.
Market Response
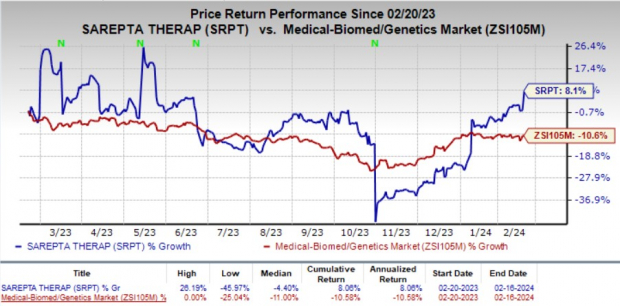
Notably, Sarepta’s stock experienced a 7.8% surge on Feb 16, reflecting overwhelming positivity and investor confidence in the company’s future trajectory. Over the past year, SRPT shares have exhibited resilience, achieving an 8.1% gain despite a broader industry decline of 10.6%.
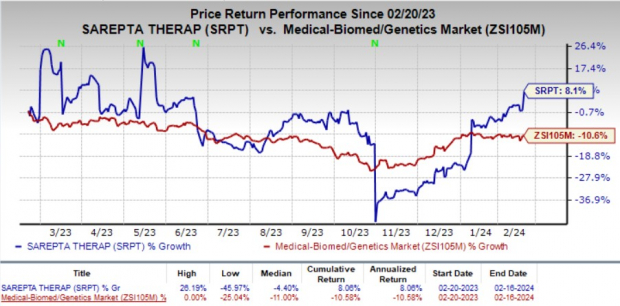
Image Source: Zacks Investment Research
Impact of EMBARK Study
The BLA supplement is predominantly underpinned by data from the phase III EMBARK study, disclosed in October 2023. Despite falling short of its primary endpoint, the study achieved statistical significance on all pre-specified key secondary endpoints. This substantial evidence suggests that Elevidys has the potential to modify the course of DMD, ushering in a new era of DMD treatment and management.
Commercial Performance
Sarepta recently released encouraging unaudited results for the fourth quarter and full-year 2023, disclosing an anticipated $131.3 million in Elevidys sales for the fourth quarter. This brings the total sales figure to approximately $200.4 million for the full year, underscoring a remarkable commercial launch for a treatment introduced just the previous quarter.
Strategic Collaboration
SRPT developed Elevidys in collaboration with Roche (RHHBY), with the two entities joining forces via a licensing agreement in 2019 to jointly develop and commercialize Elevidys. The exclusive rights granted to Roche for the ex-U.S. markets stand as a testament to the global potential of Elevidys and illuminate Sarepta’s foray into the international treatment landscape.
Future Prospects
To meet regulatory prerequisites for Elevidys’ approval outside the United States, Sarepta is actively evaluating the safety and efficacy of Elevidys in the ongoing phase III ENVISION study in non-ambulatory and ambulatory DMD patients, highlighting the company’s commitment to global accessibility and impact.
Sarepta Therapeutics, Inc. Price and Consensus

Sarepta Therapeutics, Inc. price-consensus-chart | Sarepta Therapeutics, Inc. Quote
Zacks Rank and Other Stocks to Consider
Sarepta currently holds a Zacks Rank #2 (Buy), reinforcing investor optimism and underlining the company’s promising growth trajectory within the drug/biotech industry.
For investors seeking diversified prospects, other top-ranked stocks worth considering include ADMA Biologics (ADMA) and Immunocore (IMCR), each also carrying a Zacks Rank #2 at present. The enduring appeal and performance of these stocks serve as compelling indicators of potential growth.
Zacks Names #1 Semiconductor Stock
It’s only 1/9,000th the size of NVIDIA which skyrocketed more than +800% since we recommended it. NVIDIA is still strong, but our new top chip stock has much more room to boom.
With strong earnings growth and an expanding customer base, it’s positioned to feed the rampant demand for Artificial Intelligence, Machine Learning, and Internet of Things. Global semiconductor manufacturing is projected to explode from $452 billion in 2021 to $803 billion by 2028.
See This Stock Now for Free >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
To read this article on Zacks.com click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.