Shares of Travere Therapeutics, Inc. TVTX have surged 33.3% in the past three months, surpassing the industry’s increase of 14.5%. The company completed a pre-NDA meeting with the FDA in December 2023 to discuss confirmatory data from the phase III PROTECT study, evaluating its marketed drug, Filspari (sparsentan) for the treatment of IgA nephropathy (“IgAN”), a rare progressive kidney disease.
Regulatory Achievements
Following positive feedback from the FDA, Travere is set to submit a supplemental new drug application (“sNDA”) aiming to convert the existing accelerated approval for Filspari to full approval for treating IgAN. This submission is expected in the first quarter of 2024. Additionally, the company announced a strategic reorganization focused on cost-saving measures.
As part of the reorganization, Travere aims to reduce its current workforce by almost 20%, leading to an estimated annualized savings of around $25 million starting in 2024. These strategic moves have significantly contributed to the stock’s upward trajectory in recent months.
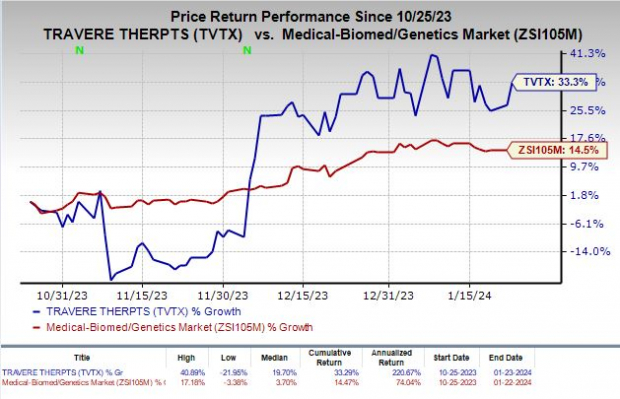 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
Expansion Plans and Milestones
Travere is also evaluating sparsentan in the phase III DUPLEX study to address focal segmental glomerulosclerosis (“FSGS”). The company plans to conduct additional analyses of the DUPLEX study data and re-engage with the FDA later in 2024 to determine the regulatory path forward for sparsentan in FSGS.
In a significant move, the company initiated the pivotal phase III HARMONY study in December 2023, evaluating its pipeline candidate, pegtibatinase, a novel investigational enzyme replacement therapy for the treatment of classical homocystinuria (HCU).
Travere, in collaboration with CSL Vifor, awaits an opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use concerning a potential approval of the conditional marketing authorization application for sparsentan in IgAN in Europe in the first quarter of 2024. The company’s progress with the Filspari sNDA, along with the drug’s strong demand among physicians, is expected to sustain the upward momentum for Travere in 2024.
Stock Performance and Market Outlook
Travere currently carries a Zacks Rank #2 (Buy). Some other top-ranked stocks in the healthcare sector include Regeneron Pharmaceuticals, Inc. REGN, CytomX Therapeutics, Inc. CTMX, and Puma Biotechnology, Inc. PBYI, each sporting a Zacks Rank #1 (Strong Buy) at present.
Over the past 60 days, estimates for Regeneron’s 2024 earnings per share have improved, while shares of REGN have rallied 28.8% in the past year, with a consistent trend of beating earnings estimates. On the other hand, CytomX Therapeutics delivered a four-quarter earnings surprise of 45.44%, on average, despite a plunge in share prices over the past year.
Puma Biotechnology also saw positive estimate revisions for 2024 earnings per share, with a rise of 9.2% in share prices over the past year, and a consistent average earnings surprise of 76.55%. This demonstrates the potential of the healthcare sector as an investment opportunity in the coming year.
With promising developments in their drug pipeline and regulatory milestones on the horizon, Travere’s strategic advancements position the company for sustained growth and potential market outperformance.