Vanda Pharmaceuticals Inc. VNDA faced a significant blow as the FDA dealt a harsh rejection, issuing a complete response letter for its new drug application (NDA) related to tradipitant, a potential therapy for symptoms of gastroparesis.
The aftermath was brutal, with VNDA stock plummeting by 6.1% on Sept. 19, echoing the disappointment among investors.
The FDA’s move comes in the backdrop of a challenging landscape, where the development of effective treatments for gastroparesis has stagnated over the past four decades.
A historical perspective reveals the urgent need for innovation in treating this condition, as existing solutions fall short in addressing the complexities of delayed gastric emptying.
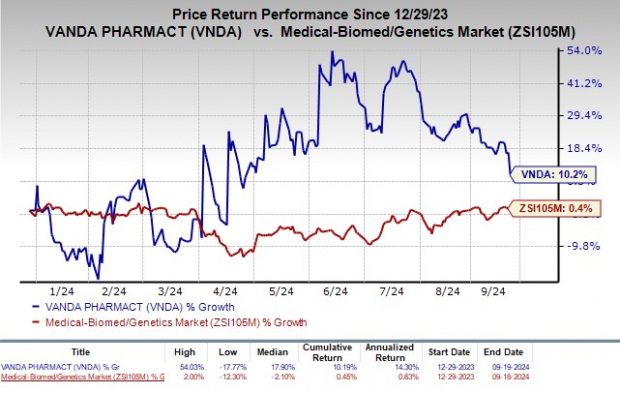
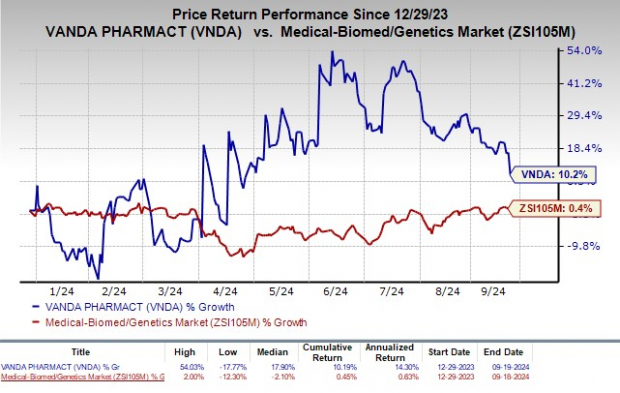
Image Source: Zacks Investment Research
Unpacking the FDA’s Complete Response Letter for Tradipitant
Within the comprehensive response letter (CRL), the FDA raised concerns that deviated from insights offered by leading experts in the field, denoting a disconnect in the evaluation process.
Moreover, Vanda highlighted issues of procedural irregularity, emphasizing the FDA’s failure to adhere to stipulated timelines mandated by the Food, Drug, and Cosmetic Act (FDCA).
Essentially, the FDA’s actions bypassed the regulatory guidelines, hinting at a bureaucratic hurdle that hindered VNDA’s progress with tradipitant.
Despite repeated efforts by Vanda to engage in productive dialogue with the FDA through an advisory committee meeting, regulatory roadblocks persisted, casting a shadow of uncertainty over the future of tradipitant.
The narrative took a poignant turn as patients reliant on tradipitant rallied behind the cause, filing a Citizen Petition urging the FDA to reconsider its stance and expedite the approval process.
VNDA’s Strategic Outlook Post FDA Setback
Following the FDA’s setback, Vanda remains undeterred in its quest to secure market approval for tradipitant to address the unmet needs of patients grappling with gastroparesis.
Moreover, the company shifts focus towards exploring tradipitant’s potential in countering vomiting induced by motion sickness, expanding the scope of its therapeutic applications.
A silver lining emerged in May 2024, as Vanda unveiled positive trial results from a phase III study targeting motion sickness-induced vomiting, signaling a promising avenue for future developments.
The roadmap ahead involves a detailed submission of an NDA to the FDA for tradipitant’s role in motion sickness treatment, slated for later in 2024, underscoring Vanda’s commitment to advancing innovative healthcare solutions.
Evaluating Vanda’s Position and Alternatives in the Biotech Landscape
Within the biotech sector, Vanda currently carries a Zacks Rank #4 (Sell), signaling a cautious stance amid prevailing challenges.
Amidst this landscape, investors might pivot towards alternative opportunities within the sector, such as strong contenders like Illumina, Inc., Krystal Biotech, Inc., and Fulcrum Therapeutics, Inc., boasting a Zacks Rank #1 (Strong Buy).
Noteworthy shifts in outlook and performance metrics underscore the resilience of these alternatives, with a trajectory that resonates positively among investors seeking potential growth avenues.
Amidst dynamic market conditions, strategic diversification and informed choices are vital for navigating the biotech terrain effectively, ensuring a balanced investment portfolio.
To read this article on Zacks.com, click here.
Market News and Data brought to you by Benzinga APIs